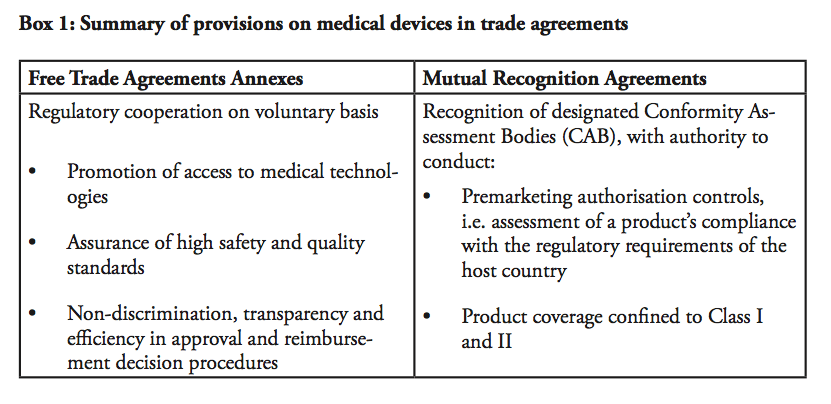
Chapter 7 of the eu gmps 7 10 through 7 15 contracts requires a quality agreement to define the responsibilities of the contract giver and contract acceptor contract manufacture and.
Medical device contract manufacturing quality agreement.
Medical device directive 93 42 eec including directive 2007 47 ec requires that the manufacturer of medical devices keeps a product related adequate and efficacious quality system.
Testing agreement for medical devices gmp quality contract.
Technical quality agreement for contract manufacturing technical agreement quality agreement.
Contract manufacturing general agreement.
Show per page.
View as grid list.
This manufacturing agreement the agreement is entered into as of this 18th day of may 2005 by and between tissuelink medical inc a delaware corporation having its principal place of business at 1 washington center suite 400 dover nh 03820 the customer and phase ii medical manufacturing inc having its.
This agreement is made and entered into on day of month year by and between seller the seller a company located at seller address and distributor the distributor a company located at distributor s address.
Quality agreements are required by medical device regulations 21 cfr 820 50 purchasing controls as well as various quality standards such as iso.
A the seller manufactures certain medical device products the products as defined in appendix 1.
All the elements requirements and provisions adopted by the manufacturer for his quality system must be documented in a systematic and orderly manner in the form.