Our maturity in medical device development design cycle brings in traceability in all process there by easier regulatory approvals and faster time to market.
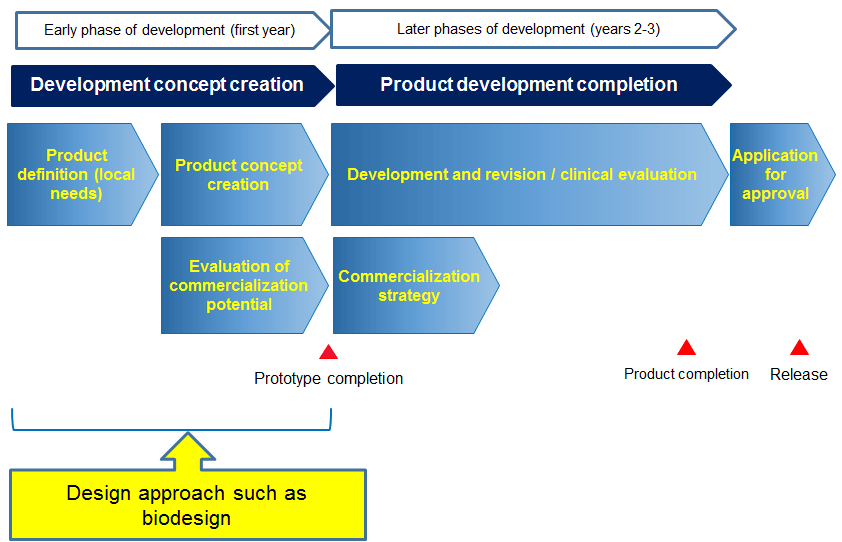
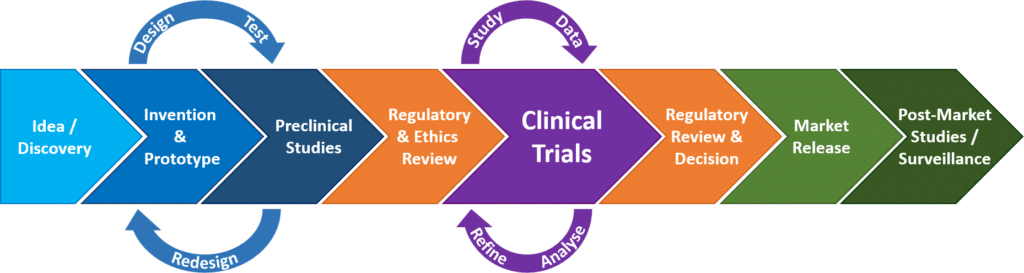
Medical device development from prototype to regulatory approval.
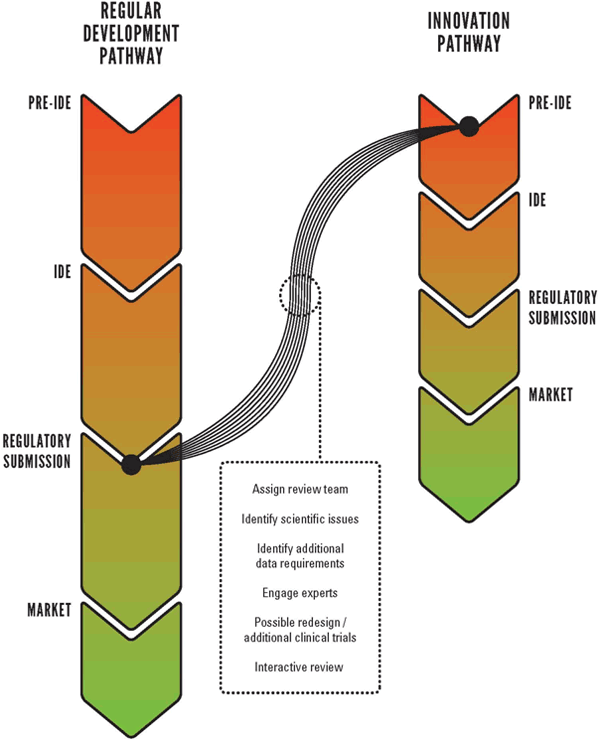
The dartmouth device drug development symposium was held in october 2003 with joint inventor entrepreneur industry clinical and regulatory participation in an effort to characterize the process by which new interventional devices are currently developed and approved.
First you need to understand how medical device development from prototype to regulatory approval works.
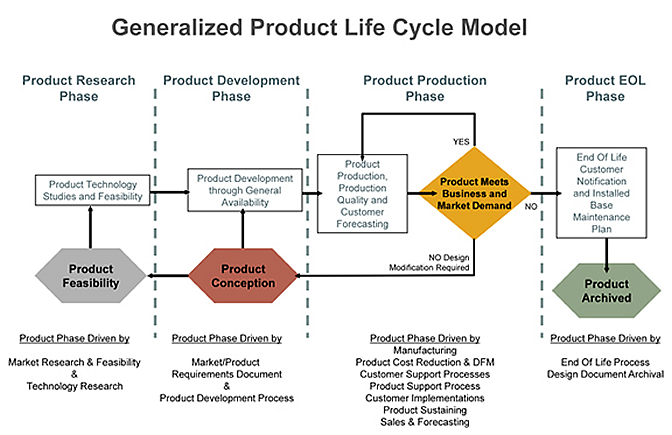
Medical device product launch.
In the early part of the 20th century the u s.
Our experts train hospital and critical care staff in device.
Since cardiac medical devices are created to help.
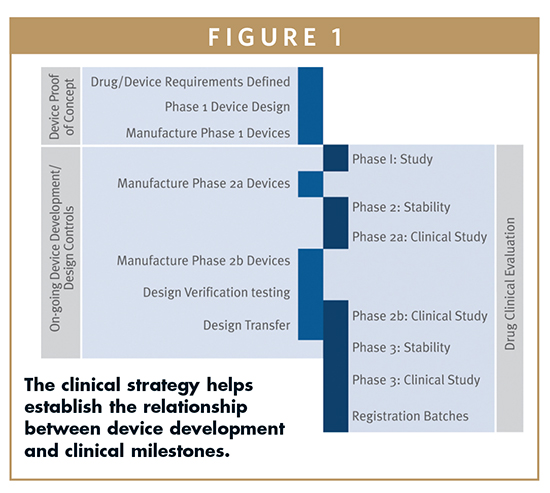
Regulatory7 path outlined clear regulatory path fda approval major company acquisition quality work risks milestone based planning risk 1 10 risk 1 10 value revenues risk 1 10.
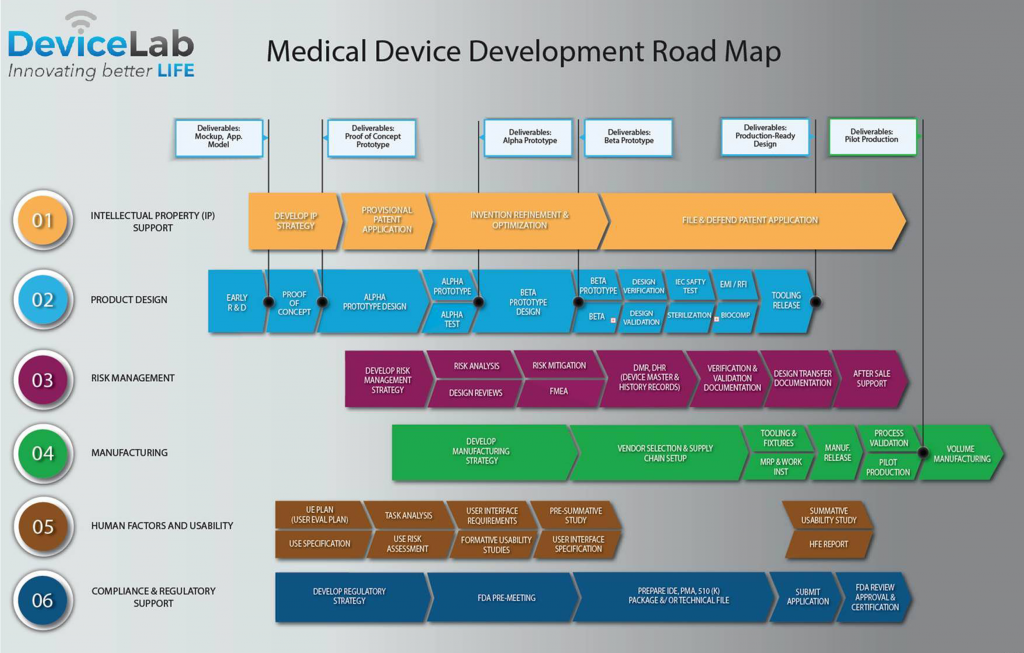
The path to commercialization of a medical device is long expensive and takes an engineering mindset.
Confidential not for circulation.
At least according to the authorities assessing market approval.
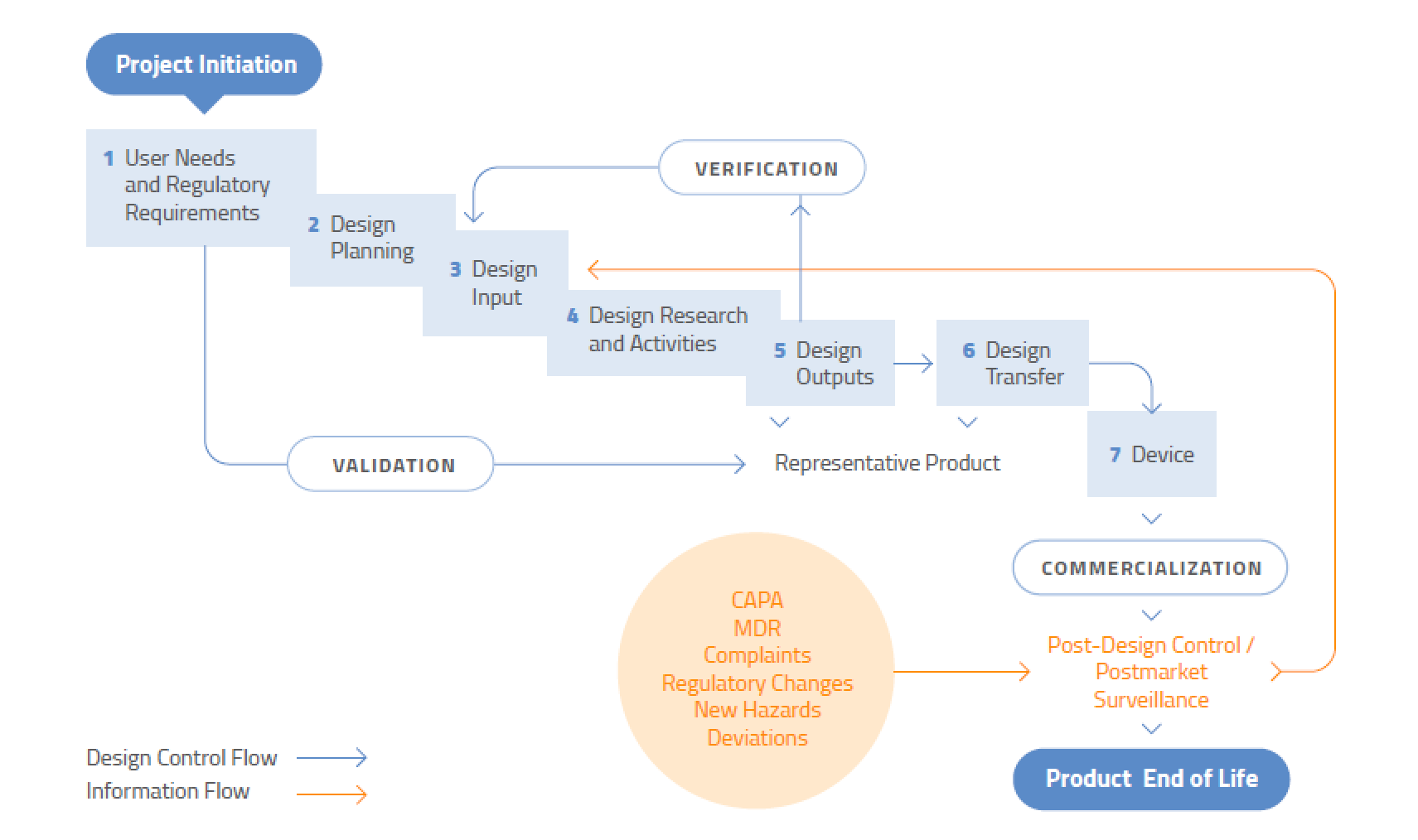
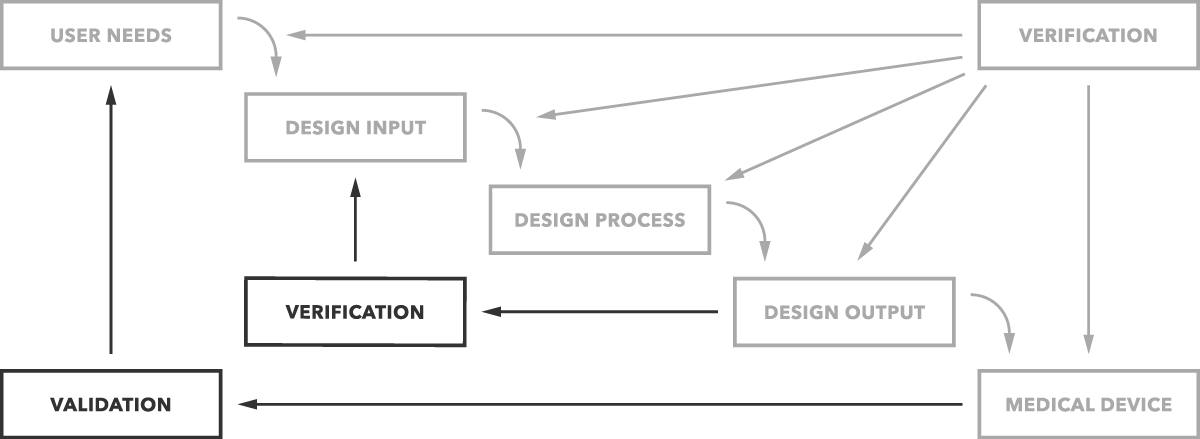
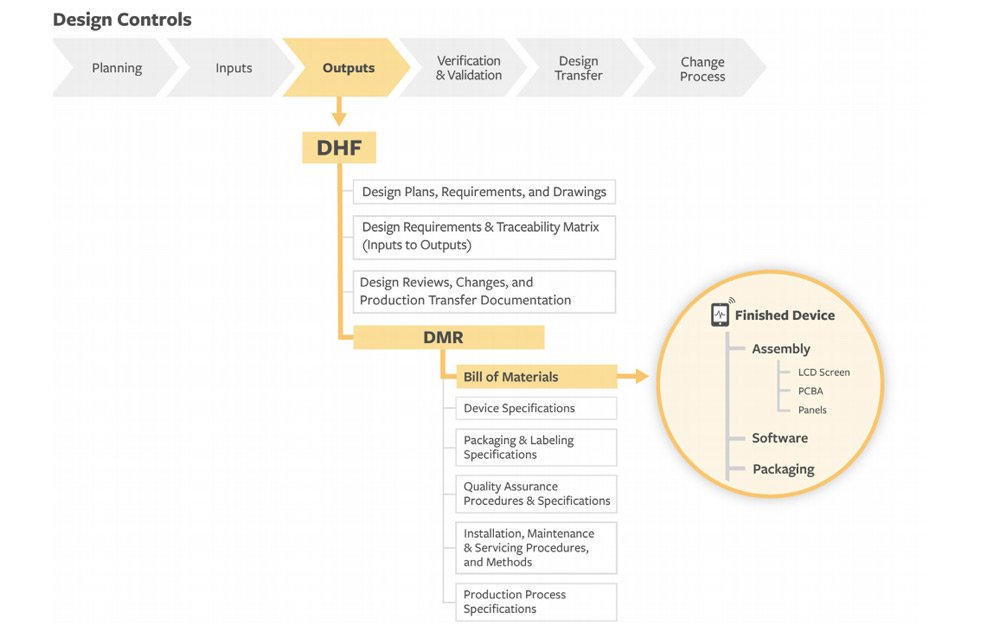
Strict design controls for the medical device industry are in place meaning an expert team is not enough to get regulatory approval for a medical device.
Food and drug administration fda was given the responsibility for ensuring both the safety and efficacy of drugs prior to marketing amendments to the federal food drug and cosmetics act in 1976 expanded the agency s role to oversee safety in the development of medical devices whereas new drug approval takes an average of 12 years moving new.
They must use a formal medical device development process with activities focused on design control.
The development path follows a certain route from device conception intellectual property generation and testing to regulatory approval.
Medical device development from prototype to regulatory approval.