2 to assist center reviewers in their review and evaluation of medical device patient labeling to help make it understandable to and usable by patients or family members or other lay persons.
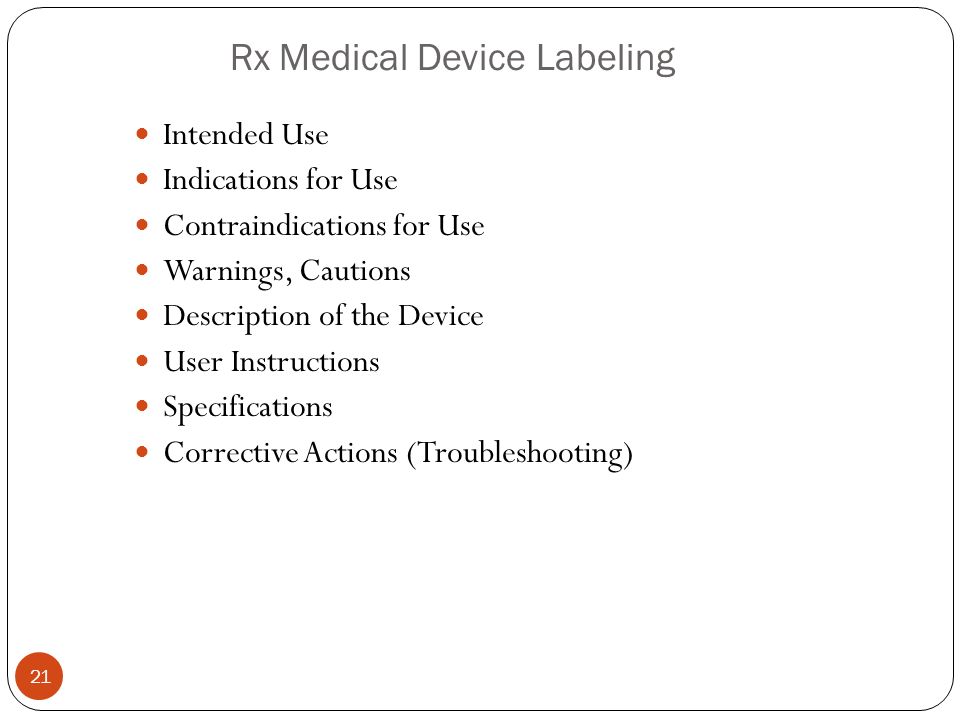
Medical device labeling suggested format and content.
How the device should be used maintained and.
Food and drug administration fda develops and administers regulations under authority granted by laws passed by congress that.
Food and drug administration fda develops and administers regulations under authority granted by laws passed by congress that.
The medium format content legibility and location of the label and instructions for use should be appropriate to the particular device its intended purpose and the technical knowledge experience education or training of the intended user s.
Introduction to medical device labeling label vs.
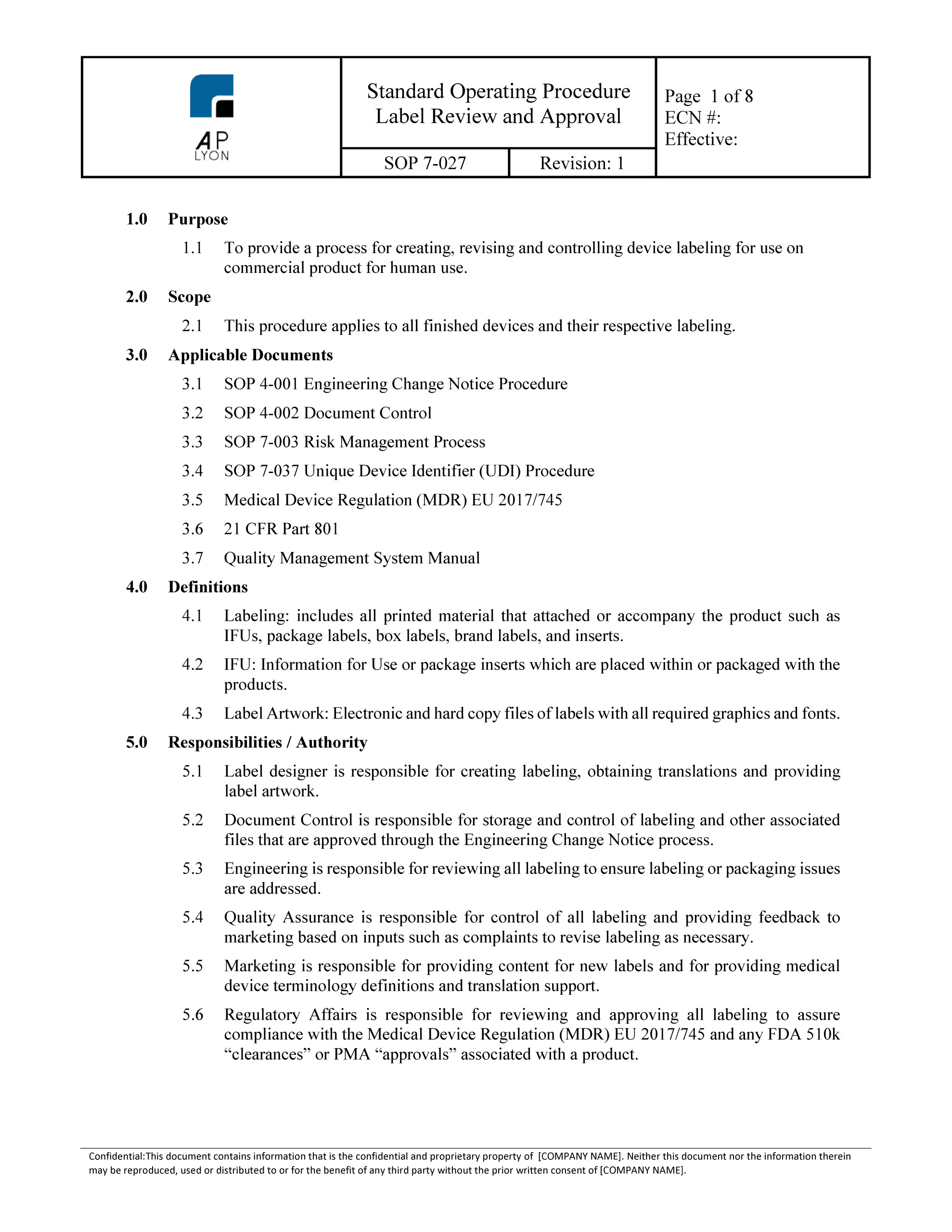
This 67 document specifies the general labeling principles including specific sections on the label 68 instructions for us and information intended for the patient.
Free labels wide collections of all kinds of labels pictures online.
Fda provides guidance to manufacturers and ras on the content of the label and the instructions for use that provide users both professional and lay as appropriate and or patients and any relevant third parties with information such as.
The device s intended use purpose.
Make your work easier by using a label.
Medical device patient labeling is supplied in many formats for example as patient brochures patient leaflets user manuals and videotapes.
Providing regulatory submissions for medical devices in electronic format submissions under section 745a b of the federal food drug and cosmetic act draft guidance for industry and food and.
Introduction to medical device labeling label vs.
The format content and location of labelling should be appropriate to the particular device and its intended purpose.
66 the general content and format of medical device and ivd medical device labeling.
Medical device labeling suggested format and content tuesday june 19th 2018.

.jpg)